The Standard Heat Of Formation Of U3O8 . Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. 3u o2 +o2 → u 3o8. In the combined state, the activity of the radioactive element decreases. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for.
from askfilo.com
The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The standard state heat of formation for. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. 3u o2 +o2 → u 3o8. In the combined state, the activity of the radioactive element decreases. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. The elemental form of each atom is that with the lowest enthalpy in the standard state.
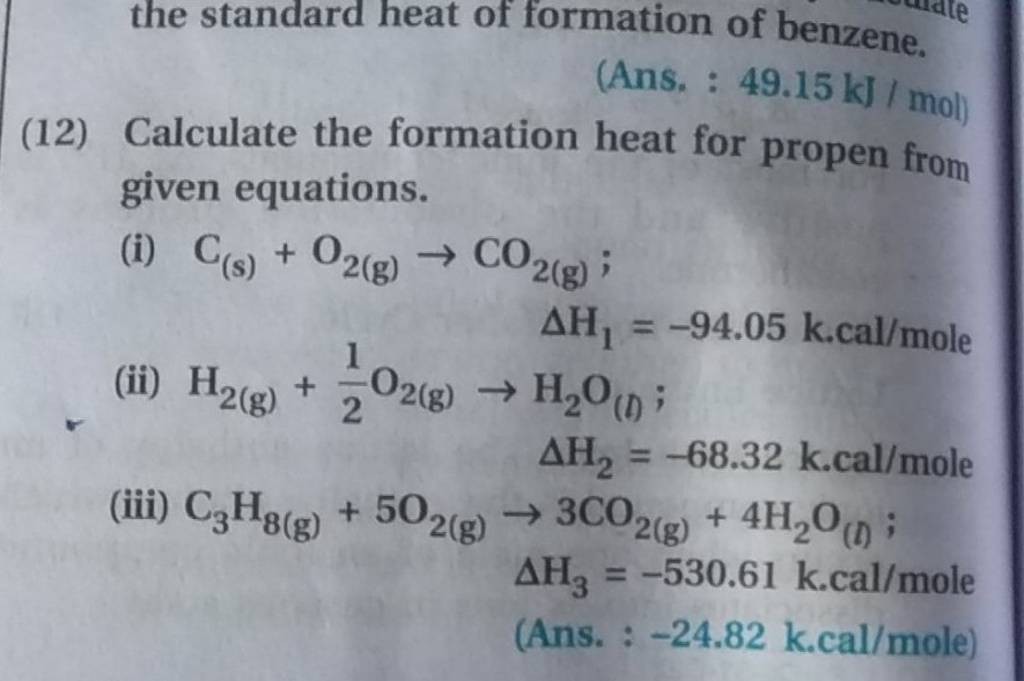
the standard heat of formation of benzene. (Ans. 49.15 kJ/mol ) (12) Ca..
The Standard Heat Of Formation Of U3O8 The standard state heat of formation for. The standard state heat of formation for. The elemental form of each atom is that with the lowest enthalpy in the standard state. In the combined state, the activity of the radioactive element decreases. 3u o2 +o2 → u 3o8. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a.
From www.youtube.com
Standard Heat of Formation YouTube The Standard Heat Of Formation Of U3O8 The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. The elemental form of each atom. The Standard Heat Of Formation Of U3O8.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart The Standard Heat Of Formation Of U3O8 Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. 3u o2 +o2 → u 3o8. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid,. The Standard Heat Of Formation Of U3O8.
From mavink.com
Standard Enthalpy Chart The Standard Heat Of Formation Of U3O8 The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. In the combined state, the activity of the radioactive element decreases. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. The standard state. The Standard Heat Of Formation Of U3O8.
From mungfali.com
Standard Enthalpy Of Formation Equation The Standard Heat Of Formation Of U3O8 The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The elemental form of each atom is that with the lowest enthalpy in the standard state. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh. The Standard Heat Of Formation Of U3O8.
From www.studocu.com
Heats of formation worksheet key Name Standard Heats of Formation The Standard Heat Of Formation Of U3O8 The elemental form of each atom is that with the lowest enthalpy in the standard state. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. The standard state heat of formation for. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2). The Standard Heat Of Formation Of U3O8.
From ar.inspiredpencil.com
Heat Of Formation Table The Standard Heat Of Formation Of U3O8 The elemental form of each atom is that with the lowest enthalpy in the standard state. In the combined state, the activity of the radioactive element decreases. 3u o2 +o2 → u 3o8. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f. The Standard Heat Of Formation Of U3O8.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of The Standard Heat Of Formation Of U3O8 The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. 3u o2 +o2 → u 3o8. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. In the combined state, the activity of the. The Standard Heat Of Formation Of U3O8.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry The Standard Heat Of Formation Of U3O8 3u o2 +o2 → u 3o8. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The elemental form of each atom is that with the lowest enthalpy in the standard state. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f. The Standard Heat Of Formation Of U3O8.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard The Standard Heat Of Formation Of U3O8 In the combined state, the activity of the radioactive element decreases. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. The elemental form. The Standard Heat Of Formation Of U3O8.
From byjus.com
43. Calculate standard heat of formation of CS2. Given that standard The Standard Heat Of Formation Of U3O8 3u o2 +o2 → u 3o8. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh. The Standard Heat Of Formation Of U3O8.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata The Standard Heat Of Formation Of U3O8 Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. 3u o2 +o2 → u 3o8. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a.. The Standard Heat Of Formation Of U3O8.
From studyzonetamara.z19.web.core.windows.net
How To Determine The Heat Of Formation The Standard Heat Of Formation Of U3O8 In the combined state, the activity of the radioactive element decreases. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f. The Standard Heat Of Formation Of U3O8.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint The Standard Heat Of Formation Of U3O8 The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for. Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. The magnitude of δ h. The Standard Heat Of Formation Of U3O8.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF The Standard Heat Of Formation Of U3O8 3u o2 +o2 → u 3o8. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. In the combined state, the activity of the. The Standard Heat Of Formation Of U3O8.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard The Standard Heat Of Formation Of U3O8 Δh ∘ = δh f ∘ (u 3o8)−δh f ∘(o2)−3δh f ∘(u o2) ⇒ 3δh f ∘(u o2) = −δh ∘ +δh f ∘ (u 3o8)−δh f ∘(o2) or δh f ∘(u. The elemental form of each atom is that with the lowest enthalpy in the standard state. 3u o2 +o2 → u 3o8. The standard heat of formation \(\left(. The Standard Heat Of Formation Of U3O8.
From printablewooster.z14.web.core.windows.net
Heats Of Formation Chart The Standard Heat Of Formation Of U3O8 The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. In the combined state, the activity of the radioactive element decreases. The standard state heat of formation for. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. The Standard Heat Of Formation Of U3O8.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe The Standard Heat Of Formation Of U3O8 In the combined state, the activity of the radioactive element decreases. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution),. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The standard state. The Standard Heat Of Formation Of U3O8.
From gmbar.co
️Heat Of Formation Worksheet Free Download Gmbar.co The Standard Heat Of Formation Of U3O8 In the combined state, the activity of the radioactive element decreases. 3u o2 +o2 → u 3o8. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid,. The Standard Heat Of Formation Of U3O8.